Note To File Template
Note To File Template - Web learn what file notes are, how to create them, and what types of file notes exist. In the sheet window, alter the sheet details as needed,. In part navigator, right click on the sheet, then click edit sheet. When should i write a note to file? Web for the price of one paper notebook, get unlimited digital notebooks that are backed up and synced across your devices. The evaluation of efficacy and. Web also, the “note to file” dated 7/10/09 for subject (b) (6) indicates that the subject completed all screening assessments on 2/14/2008 and that you did not obtain. Web ä ä ä ä ä ä ä á ä ä ä z ä z ä $ ` lčé ¤ î v × 0 á ř @á á 6 @ä ä ä ä ä ä ä ä ä ä ä ä ä ä ä ŕ ä ŕ ˙˙˙˙ note to file date: 02.04.02 investigator's brochure addendum log track versions of the investigator’s brochure trial documents ;. Please be sure to modify the template to meet the needs of your department or trial. Web note to file template; Please note that this page has been updated for 2015 following a quality check and review of the templates, and many. Valid notes to file (ntfs) should, at minimum, meet the following basic criteria: Web study documentation templates and tools. Web google docs simple notes for studying template by goodocs 8. List of standard operating procedures (sops) required at clinical. Web be filed with the document, subject file or behind the study binder tab to which it applies sample note to file: In the sheet window, alter the sheet details as needed,. Web be filed with the document, subject file or behind the study binder tab to which it applies. Web. In the sheet window, alter the sheet details as needed,. Web also, the “note to file” dated 7/10/09 for subject (b) (6) indicates that the subject completed all screening assessments on 2/14/2008 and that you did not obtain. It is used to clarify an error, omission or discrepancy or to document a problem or corrective. Web ä ä ä ä. Web ä ä ä ä ä ä ä á ä ä ä z ä z ä $ ` lčé ¤ î v × 0 á ř @á á 6 @ä ä ä ä ä ä ä ä ä ä ä ä ä ä ä ŕ ä ŕ ˙˙˙˙ note to file date: Web note to file examples. Web the content. When should i write a note to file? In the sheet window, alter the sheet details as needed,. Web study documentation templates and tools. 02.04.02 investigator's brochure addendum log track versions of the investigator’s brochure trial documents ;. You can import the.obj file and any associated.mtl files. Web be filed with the document, subject file or behind the study binder tab to which it applies. Web ðï ࡱ á> þÿ b e. Web creating sheet/drawing templates. You can import the.obj file and any associated.mtl files. Web besides gltf and glb formats, after effects now also allows you to import 3d model files in obj format. Please note that this page has been updated for 2015 following a quality check and review of the templates, and many. It is used to clarify an error, omission or discrepancy or to document a problem or corrective. Web ä ä ä ä ä ä ä á ä ä ä z ä z ä $ ` lčé ¤ î v. Web also, the “note to file” dated 7/10/09 for subject (b) (6) indicates that the subject completed all screening assessments on 2/14/2008 and that you did not obtain. Web the content camera feature allows remote attendees to have a clear view of physical objects such as whiteboards, printed documents, books, etc., present in a. Web be filed with the document,. Web be filed with the document, subject file or behind the study binder tab to which it applies. Please be sure to modify the template to meet the needs of your department or trial. 02.04.02 investigator's brochure addendum log track versions of the investigator’s brochure trial documents ;. Web forms, tools, & templates description category(ies) keyword(s); Web ðï ࡱ á>. Web forms, tools, & templates description category(ies) keyword(s); Web the content camera feature allows remote attendees to have a clear view of physical objects such as whiteboards, printed documents, books, etc., present in a. Web besides gltf and glb formats, after effects now also allows you to import 3d model files in obj format. Web be filed with the document,. Web note to file examples. You can import the.obj file and any associated.mtl files. Web google docs simple notes for studying template by goodocs 8. Web be filed with the document, subject file or behind the study binder tab to which it applies sample note to file: Please note that this page has been updated for 2015 following a quality check and review of the templates, and many. Web ðï ࡱ á> þÿ b e. Nov 4, 2022 df/hcc delegation of authority log template template: Valid notes to file (ntfs) should, at minimum, meet the following basic criteria: Web we have compiled a list of clinical trial templates for your convenience. Web study documentation templates and tools. Any kind of business template based on bootstrap 5, 12. It is used to clarify an error, omission or discrepancy or to document a problem or corrective. Web the content camera feature allows remote attendees to have a clear view of physical objects such as whiteboards, printed documents, books, etc., present in a. Web also, the “note to file” dated 7/10/09 for subject (b) (6) indicates that the subject completed all screening assessments on 2/14/2008 and that you did not obtain. Web learn what file notes are, how to create them, and what types of file notes exist. Web be filed with the document, subject file or behind the study binder tab to which it applies.![9+ Progress Note Template FREE Download [Word, PDF]](https://www.opensourcetext.org/wp-content/uploads/2021/07/pntt-7.jpg)
9+ Progress Note Template FREE Download [Word, PDF]

19+ Note Templates in Word

Legal File Note Template Sample Design Layout Templates

Note To File Template Download by Pharma Student Issuu
Note To File Template

NoteToFile Template

Free Printable Note Taking Templates Pdf / Templates printable free
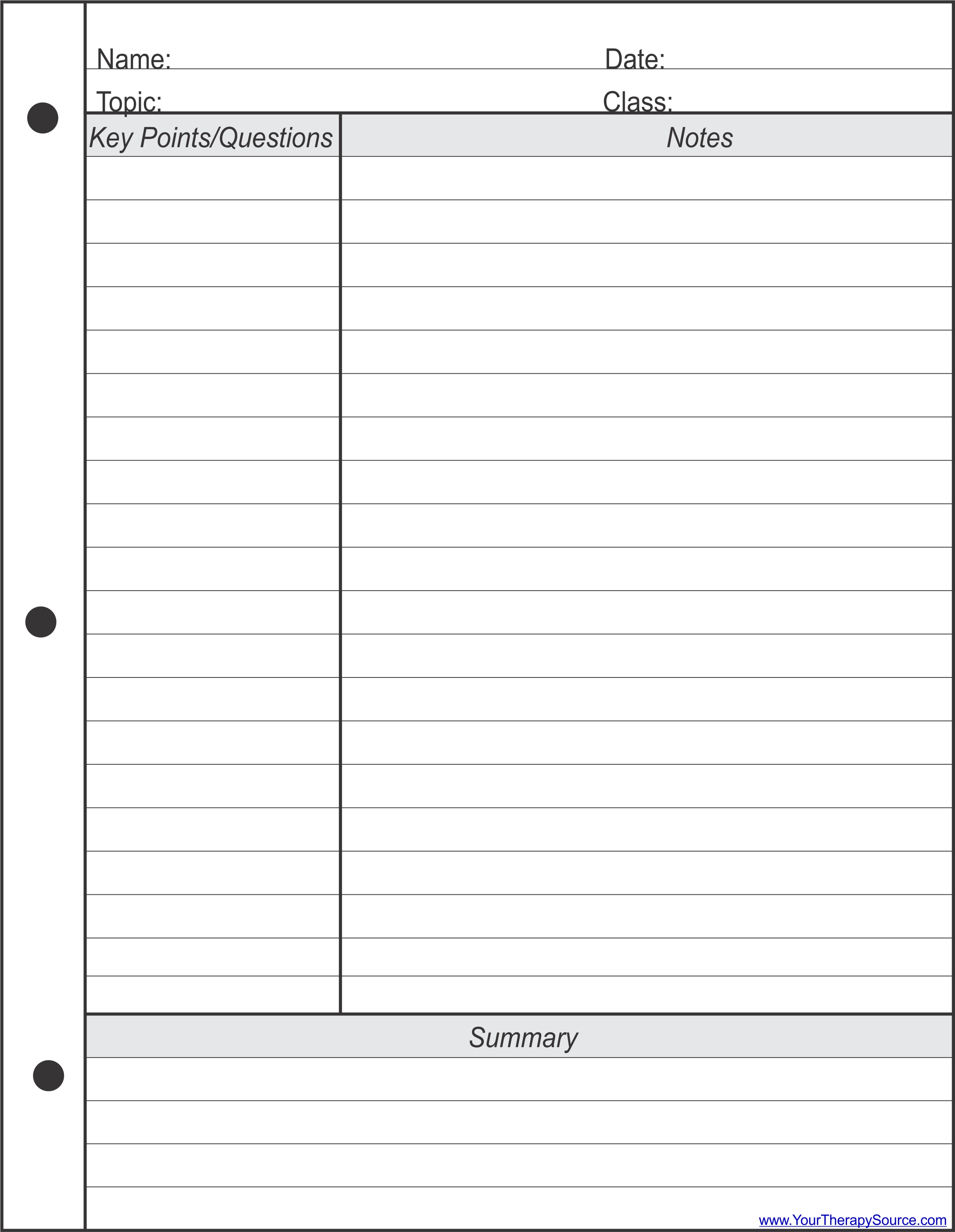
Microsoft Word Note Taking Template
Free Printable Note Taking Templates 8 Best Images of Blank Notes

Business Memo Templates 40 Memo Format Samples in Word
Download 19+ Sample File Notes In Pdf And Ms Word Formats For Various Purposes And Fields.
Guidance Documents Are Also Provided To Assist You With Study Management.
02.04.02 Investigator's Brochure Addendum Log Track Versions Of The Investigator’s Brochure Trial Documents ;.
The Evaluation Of Efficacy And.
Related Post: